Our daily starch ((C6H10O5)n, CAS nr. 9005-25-8) aka amylum that has been used for millennia for everyday energy to enjoy at our tables and for centuries to strengthen our garments, paper, board and magazines, was assigned a somewhat different purpose by Mother nature. Let’s consider some fundamental aspects of the biopolymer starch, its sources and structure.
Basically, all green plants utilize the electromagnetic energy (photons) in sunlight and the basic molecules CO2 and H2O in their chlorophyll to chemically ‘build themselves life’ by self-generating carbohydrate molecular structures via photosynthesis. This is the common principle of all living plant tissue. The ingenious chemistry in these refined biological processes and the carbohydrates (the class of chemical compounds initially named by their principal structure: Cx(H2O)y) are key sources for life on Earth. Plants grow by cellular production of cellulose, starch and less complex carbohydrates together with proteins. These molecular compounds are providing structural rigidity, energy and more complex functionalities, respectively. Every living thing needs chemical energy in some form. Starch is an ingenious form of chemically stored energy found in vegetation. It can be available both for the plant itself and for the next generation in seeds, tubers or bulbs.
Mankind knew that some plants were edible, some likely found via the animal kingdom, and we have gradually learnt to prepare them into more and more advanced food. Initially mainly for energy purposes, secondly for taste, preservation and convenient handling. However, recently food and taste dimensions have surmounted as our standards of living, on average, have elevated. Starches represent the largest amount of carbohydrate we consume via vegetable staple foods, such as cereals, tubers and fruits. You have most likely been using starch in some food making in your kitchen. The food grade products normally used are in form of a dry, white and light powdery stuff. The natural sources here used can be maize, potato, tapioca and various cereals. If one’s not careful this powder tends to leave traces all over the place – one better leave-out sneezing while spooning over some starch… These powders consist of starch granules with a molecular configuration highly packed into spherical structures. Generally, the natural starch shows a low osmotic activity implying that the natural way of packing starch does not impair the cellular water balance in the plants. Moreover, starch in native shape has a crystalline structure containing different amounts of bound water depending on if we are dealing with cereal- or tuber-based starches. These packages contain a high amount of biological/chemical energy concentrated into a small volume. Starch in edible forms are real energy packs, as we know from eating some in its natural packaging.
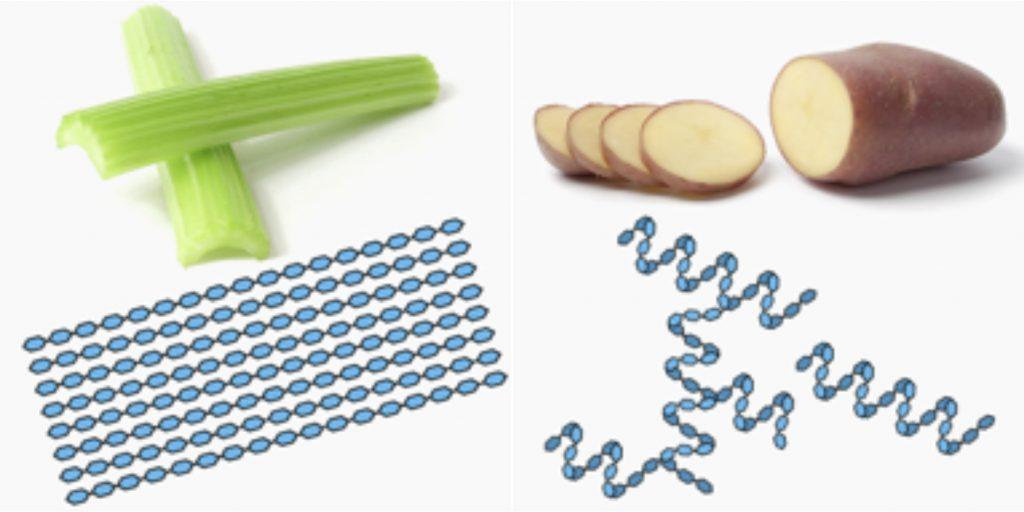
On average, there is 1/5th to 1/4th of linear amylose in natural starches with the remaining part being branched amylopectin. The cereals normally contain a fraction of various proteins while tubers do not. Starch could be considered an α-relative, why not even sibling, to the β-bonded structural polysaccharides cellulose, chitin and peptidoglycan. The amylose form of starch has helical molecular structures, not linear as in the natural building materials, e.g. cellulose. The molecular chains in cellulose are strongly associated via numerous hydrogen bonds, both inter- and intra-molecular. This is the main reason behind the high physical durability of cellulose that we experience in plant tissue, fibers and wood.
The native starch granules normally tend to be insoluble in water at room temperature, mainly forming an opaque dispersion. The oblong granules display a peculiar rheology as we move to higher dry solids, called dilatancy. In this form the dispersed granules even allow for odd activities like running or “biking on starch.” https://www.youtube.com/watch?v=BleCJJAKkgw
When heated to above 60°C the starch granules swell as water is successively being irreversibly absorbed in the granular microstructures turning the starch-water mixture transparent. We utilize this in our kitchen when preparing sauces, creams and puddings, all with viscous textures. Also the common ”al dente” sensation traditionally desired in our (wheat) pasta is arising from partial swelling of starch structures within the coagulated protein fibrillar network.
When freed up and dissolved (cooked), the helical molecular chains in amylose starch will tend to re-associate and self-assemble with time, especially when cooled. As the starch chain are re-aligned, even a stronger re-association into crystalline structures may take place. Here the formation of hydrogen bonds occurs. We refer to this phenomenon as starch retrogradation which is another phenomenon frequently encountered in the kitchen. In practice, these events of reassembly can be observed as a gelling of starch. Other related retrogradation phenomena is staling of bread and buns by time – we all know how bread turns harder and the wheat buns loose softness when stored a few days. These also involve bridging of starch chains. Our basic foods breakfast cereals, parboiled rice, rice stick noodles, croutons and bread crumbs have been subject to a technical retrogradation-type process for achievement of the desirable texture.
When dry starch is heated we can get dextrin. This a modified i.e. degraded and highly water-soluble form of starch that with reduced viscosity arising from shortened starch-chains with a higher branching degree. This was discovered by accident when a British textile mill burned down in 1821 when the brownish, pyrolyzed starch was observed to dissolve easily in the firemen’s water while also tasting sweet. When G.S.C. Kirchoff subjected starch to heat in mild acidic conditions in aqueous environment he invented the basis for the starch-based sweetener industry while preparing starch syrup in the early 19th century. Some dextrines color red by iodine in opposite to starch that goes blue in iodine solution.
The starch chain is interconnected via α-1-4 by glyosidic bonds between the sugar units; here every second glucosidic group is rotated 180°, when viewed along the chain axis. Starch may have molar weights up to millions. These acetal bonds can be cleaved even by mild acids. Our gastric mix contains strong acid having a pKa<1 and can easily hydrolyze various starches – and that’s why we can digest starch without problems. Most of the β-bonded carbohydrate structures are clearly not directly intended for our intestinal systems to break apart by acid hydrolysis into sugar units. Occasionally we may get some biochemical help by enzymatic boosting. The ruminant animals, e.g. cows, are instead gastritically well-equipped for handling the sturdier carbohydrates like cellulose – making these no match for them.
Being a renewable biomaterial grown everywhere on our Earth makes starch a vivid raw material source for the many applications we have invented. At Chemigate we have a wide range of starch-based products available for the papermaking industry. Being the second most used carbohydrate after cellulose proves the fact stated by many people: “starch is the mother of additives in papermaking”. By principal it can be very difficult to produce a strong and good paper or board grade without starch addition to some descent price level. Papermakers can use any of the native, nonionic, anionic or cationic grades available for improvement of their process, product or performances.
Already the Egyptians sized their papyrus[1] sheets with starch around 3500 B.C. for improved ink performance. At the time, wheat starch slurry was boiled in acetic acid to provide a paste easily spread onto the sheets. This was used for sizing the different layers of papyrus sheets and for coating the final sheet to slow down ink absorption. Also the Chinese used starch, and evidence based on paper sheets from those times has indicated the usage of both wet-end and sizing starches. In Oriental paper grades from 7001300 A.D. starch granules from rice, barley and wheat have been identified.
Starch turned more popular again in the 19th-century as paper produced from wood-based sources made its entrance. The close molecular kinship between starch and cellulose makes them the best of companions when it comes to papermaking. Chemigate provides a wide range of green and high-tech starch-based products with well-documented success: from fixatives and wet-end starches to surface sizes and coating starches including dextrin-based products with high efficiency, both from economic and technical aspects.
References
Starch: Chemistry and Technology Eds.: BeMiller J. and Whistler R. 2009 ISBN: 978-0-12-746275-2
Lamberg, H. (2016) Tärkkelys ja tärkkelysteollisuus, MSc-work, JyU
https://www.writeabout.com/2014/11/starch-retrogradation-2/
http://papermakinginnovation.com/
http://www.paperonline.org/uploads/history.pdf
http://www.starch.dk/isi/applic/paper.htm
Ralph W. Kerr and Frank C. Cleveland: Chemistry of Dextrinization, 1953 DOI: 10.1002/star.19530051002
http://www.ancientegyptianfacts.com/ancient-egyptian-papyrus.html
http://sciencemeetsfood.org/starch-gelatinization/
https://dlc.dcccd.edu/biology1-3/carbohydrates
Resmini, P. and Pagani, M.A. (1983) ”Ultrastructure Studies of Pasta. A Review.,”Journal of Food Structure: Vol. 2: No. 1, Article 2.Available at: http://digitalcommons.usu.edu/foodmicrostructure/vol2/iss1/2
[1] Papyrus means ”that which belongs to the house”
TallennaTallenna

